Pure graphene is a crystal with only one atom thick and thickness of about 0135nm. It has ultra-thin, ultra-strong and super-strong thermal conductivity and excellent mechanical properties. It is expected to be used in high-performance electronic devices, composite materials and fields. It is widely used in the fields of launch materials, gas sensors and energy storage.
The basic structural unit of graphene is the most stable benzene six-membered ring in organic materials, and it is the most ideal two-dimensional nanomaterial. Graphene oxide obtained by ultrasonically stripping graphite oxide cannot be stably present under normal environmental conditions. The graphene atoms are constantly vibrating and the amplitude of the vibration may exceed their thickness. Meyer and Geim et al. showed that the structure of graphene became quite stable after fluctuating in the third dimension, especially for single-layer graphene, which reduces the surface energy and converts from two-dimensional to three-dimensional morphology. The fold is the existence of two-dimensional graphene. Necessary conditions.
Graphene has a very high thermal conductivity and has recently been advocated for heat dissipation. The embedding of graphene or several layers of graphene FLG in the heat sink can greatly reduce the local hot spot temperature. Therefore, it is necessary to conduct an in-depth study on its thermal conductivity.
The development of thermal conductivity of nanomaterials is slow, in part because of experimental testing and the difficulty of controlling heat transfer at the nanoscale. Atomic force microscopy with nanometer-scale high resolution has been used to test the thermal conduction of nanostructures, providing a feasible method for detecting the thermal properties of nanostructures, but the theoretical simulation and analysis of the heat transfer of nanostructures is still under investigation. Known viable methods, including the number solution of Fourier's law, and the analytical methods based on Boltzmann Boltzmann transport equation and molecular dynamics Molecular-dynamics (MD) simulation have their own limitations. When the size of the material drops to the nanometer scale, the temperature also becomes less stable. In a balanced system, temperature is defined based on the average energy of the material. For nanosystems such as graphene, the size of the material is too small to determine the local temperature. Therefore, the concept of temperature under equilibrium conditions cannot be applied to nanomaterials, making it difficult to conduct theoretical analysis of heat conduction at the nanoscale.
Graphene is a honeycomb two-dimensional material in which carbon atoms are sp2 hybridized, and the basic structural unit is the most stable six-membered ring in organic materials. This unique structure gives it many excellent properties, such as: high thermal conductivity, around 3000 W·(m·K)-1; excellent electrical conductivity, carrier mobility up to 2 & TImes; 105 cm2· (V·s)-1; and light weight, the theoretical surface area is 2630 m2/g, the Young's modulus is 1.0 TPa, and the mechanical properties are comparable to those of carbon nanotubes. Graphene materials are readily available, and the preparation process and processability are also improving. According to the excellent performance and low cost of graphene, it can be functionalized and used to develop various high performance polymer composite materials.
Introduction to grapheneIn 2004, Geim et al. first discovered a new two-dimensional atomic crystal, graphene (GR). They used a mechanical stripping method to successfully strip graphene from graphite with ordinary tape and observed it. So far, carbon materials have zero-dimensional fullerenes, one-dimensional CNTs, two-dimensional graphene and three-dimensional complete systems of diamond and graphite.
Graphene structureGraphene is a monoatomic layer connected by carbon atoms with sp2 hybridization, and has a honeycomb two-dimensional grid structure with a single atom thickness (theoretically only 0.35 nm), as shown in FIG.
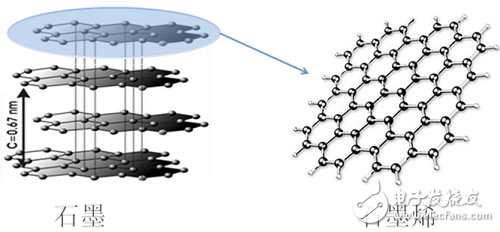
Figure 1 Schematic diagram of the GR structure
Specifically, the carbon atoms adjacent to each other in the graphene form a sigma bond, and the carbon atoms are hybridized by sp2, and a large π bond is formed based on the unbonded π electrons and the p-orbital orbital (as shown in FIG. 2). The basic structural unit of an alkene is the most stable six-membered ring in an organic material. However, graphene is not a perfect and flat two-dimensional structure film, and its surface has a large number of microscopic undulations, that is, wrinkles.

Figure 2 σ key and vertical plane π bond trajectory in GR plane
Graphene properties and applicationsThe unique structural characteristics of graphene give it a lot of excellent properties, and thus it has broad application prospects. However, the number of graphene layers obtained by general preparation is large, and the thickness is several tens of nanometers, and the performance is inferior to that of single-layer graphene.
Mechanical propertiesThe mechanical properties of graphene are highlighted by high strength and high modulus. Graphene has a strength of 130 GPa, which is about 100 times that of ordinary steel. At the same time, its tensile strength is as high as 125 GPa, the elastic modulus is 1.1 TPa, and the mass is light. The theoretical surface area is 2630 m2/g and the Young's modulus is 1.0 TPa.
The graphene hardness is higher than that of the Mohs hardness of 10 grade diamond, but it also has good toughness, can be bent, and has excellent ductility.
Electrical performanceDue to the π orbital in the plane of graphene, electrons can move freely in the crystal, and its structure is very stable. The internal carbon atoms are connected flexibly. When an external force is applied, the carbon atom plane will bend and deform, but the carbon atoms will not be rearranged. This stable lattice structure ensures excellent electrical conductivity.
The band structure is special, and the holes and electrons are separated from each other, thus causing a new electron conduction phenomenon. Novoselov et al. observed the room temperature quantum Hall effect of graphene, and the mobility of the massless Dirac-Fermi carrier was around 200,000 cm2/V·s. Heersche et al. found that graphene has superconducting properties. At the same time, graphene has outstanding properties in the bipolar electric field effect, has ballistic transmission characteristics (up to 0.3μm at 300K), and is basically immune to temperature and doping effects.
The excellent electrical properties of graphene can be applied to electronic transport devices, solar or lithium-ion batteries, supercapacitors and the like. The electrons in graphene are standard Dirac-Fermis, which makes graphene a good physical experimental platform for testing quantum electrodynamics.
Thermal performanceGraphene has excellent thermal properties, which are manifested in high thermal conductivity and negative thermal expansion coefficient. Theoretically, the thermal conductivity of single-layer graphene is as high as 6000 W/mK, and the thermal conductivity of single-layer and multi-layer graphene measured in actual experiments is about 5000 W/mK and 3000 W/mK, respectively. It can be seen that the thermal conductivity of graphene is much higher than the thermal conductivity of copper (398 W/mK), silver (427 W/mK), gold (315 W/mK) at room temperature, even more than carbon nanotubes and diamonds (2000 W/mK). )better.
Properties and applications of graphene with different slice sizesThe lateral dimension of the sheet plays an important role in controlling the microstructure and properties of the graphene-based material. In general, reducing the size distribution of the graphene sheets can improve the characteristics of the macroscopic graphene material. Whether large or small sheets have their own advantages, large layers of graphene can be used to fabricate graphene-based three-dimensional networks, 2D layered architectures, and conductive films for optoelectronic devices [10]. In these cases, the larger the graphene sheet layer, the less the joint point with other sheets, and the smaller the contact resistance. The small layer of graphene, with its more prominent electrochemically active biocompatibility, is more suitable for sensing and biological applications. Moreover, the electrical conductivity of graphene materials has a great relationship with thermal conductivity and sheet size of graphene. For example, in general, large sheets of graphene have higher electrical conductivity than small sheets.
Preparation of grapheneInitially, graphene was obtained by micromechanical stripping, but this method was time-consuming and labor-intensive and could not be mass-produced. With the increasing demand for graphene, improving its preparation method has become one of the main research goals of scholars. At present, the preparation methods of graphene generally fall into two categories: chemical methods and physical methods. Briefly introduce several types:
(1) Mechanical peeling method, the method used when graphene was first discovered. Graphene was obtained by repeatedly peeling off to a thinner graphite sheet layer directly using a transparent tape. Although this method can easily and conveniently prepare graphene, it cannot be mass-produced, and the produced graphene is difficult to control in size and has large defects.
(2) Orientation epitaxial method, which uses a growth substrate atomic structure to "speculate" a single crystal layer on the single crystal substrate to the same crystal orientation as the substrate. First, let the carbon atoms infiltrate the crucible at 2550 ° C, and then cool to 2310 ° C. The large amount of carbon atoms absorbed before form the shape of the lens, and floats out and fills the entire surface of the crucible, eventually "long" into a complete layer of graphite. Alkene. The graphene obtained by this method has high orientation, but the thickness is not uniform, and the performance is impaired.
(3) A method of chemical vapor deposition, in which a reactant is subjected to a chemical reaction in a gaseous state, and the product is solid-deposited on the surface of a solid substrate, and finally a solid product is prepared. This method is the main method for large-scale industrial production of semiconductor thin films, and the production process has been relatively perfect. Reina et al. used polycrystalline nickel as a substrate and successfully obtained a graphene film of 12 layers or even a single layer on the surface by a mixture of pyrolysis methane and hydrogen. The method can control the size of graphene sheets and can also be used for large-scale production, but the prepared graphene lacks certain properties, such as quantum Hall effect, and its electronic properties are greatly affected by the substrate.
(4) Electrochemical method. Liu et al. use a graphite rod as an electrode and an ionic solution as an electrolyte to electrochemically cleave the graphite sheet on the anode to form graphene. The method prepared by the method is graphene oxide, and the sheet layer can be well dispersed in a polar solvent and has certain conductivity.
(5) Redox method. First, natural graphite or expanded graphite powder and strong oxidizing agent and strong acid are used as raw materials to form a colloidal system, and graphene oxide is obtained after the reaction. This process is mainly used by the Hummers method. The concentrated sulfuric acid and potassium permanganate are used as oxidants to oxidize the graphite powder. The oxygen atoms enter the graphite layer and combine with the π electrons to destroy the π bond in the layer. An oxygen-containing functional group such as a carbonyl group or a carboxyl group is formed on the layer. Then, the graphene oxide is reduced to obtain graphene. Commonly used reduction methods include chemical reduction and thermal reduction. As shown in Figure 3.
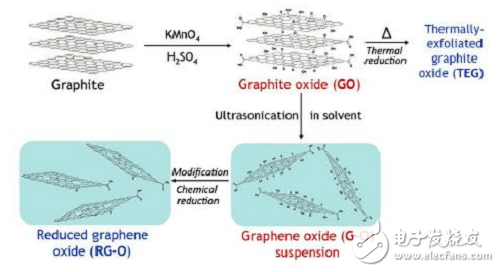
Figure 3 Flow chart of graphene prepared by redox method
Research progress in graphene compositesGraphene/polymer composites have become a hot topic in recent research based on the excellent properties of graphene. They have potential applications in electronic devices, structural materials, sensors, and biomaterials. However, research on graphene/silicone rubber composites mostly focuses on electrical conductivity, thermal conductivity, mechanical properties, etc., but there is not much research on heat resistance. At the same time, there are few discussions on the influence of graphene sheet size on composite materials.
Zhao Li et al. prepared graphene/silicone rubber composites by solution method, and studied the influence of graphene on the electrical properties and mechanical properties of silicone rubber. It was concluded that with the increase of graphene doping amount, silicone rubber composites The mechanical properties such as tensile strength and hardness are improved, and the electrical conductivity is continuously increased. Mu et al. obtained the expanded graphite/silicone rubber composite by melt mixing and solution intercalation, and studied the thermal conductivity of the composite. It was found that the thermal conductivity of the composite increased with the continuous addition of expanded graphite. And the solution intercalation method has a good effect. Hu et al. added graphene to carbon nanotube/silicone rubber composites. It was found that graphene can promote the dispersion of carbon nanotubes in the system, thereby greatly improving the mechanical properties, electrical conductivity and thermal conductivity of silicone rubber composites. Xiang et al found that graphene nanoribbons can improve the gas barrier properties and mechanical properties of composites.
With the deepening of research, scholars gradually began to explore the graphene sheets. The results show that the graphene sheet size has a great influence on the material: Jun et al. found that the larger sheet graphene sheet shows higher conductivity and makes the silicone rubber have better conductivity; Sato et al. Out, the smaller graphene sheets have stronger redox reactivity. It is worth noting that Cao et al. showed that the phonon vibration and boundary scattering in graphene affect the thermal conductivity of the material. For graphene nanoribbons, the larger the layer, the higher the phonon resonance. Strong, the lower the thermal conductivity. It can be seen that there are significant differences in the thermal conductivity of graphene of different sheet sizes, which provides a possibility to study the influence of heat conduction on the heat resistance of graphene/silicone rubber composites.
China's leading manufacturer and supplier of solar panels, all black solar panels, PERC solar panel technology in China, we specialize in solar panels, solar power systems and more.
Jinko Solar Panels, Longi Solar Panels, Canadian Solar Panels, Trina Solar Panels, JA Solar Panels, Solar Panel, Monocrystalline Solar Panel, MONO Solar Panel, Polycrystalline Solar Panel, All Black Solar Panel, 400W Solar Panel
Power X (Qingdao) Energy Technology Co., Ltd. , https://www.solarpowerxx.com
